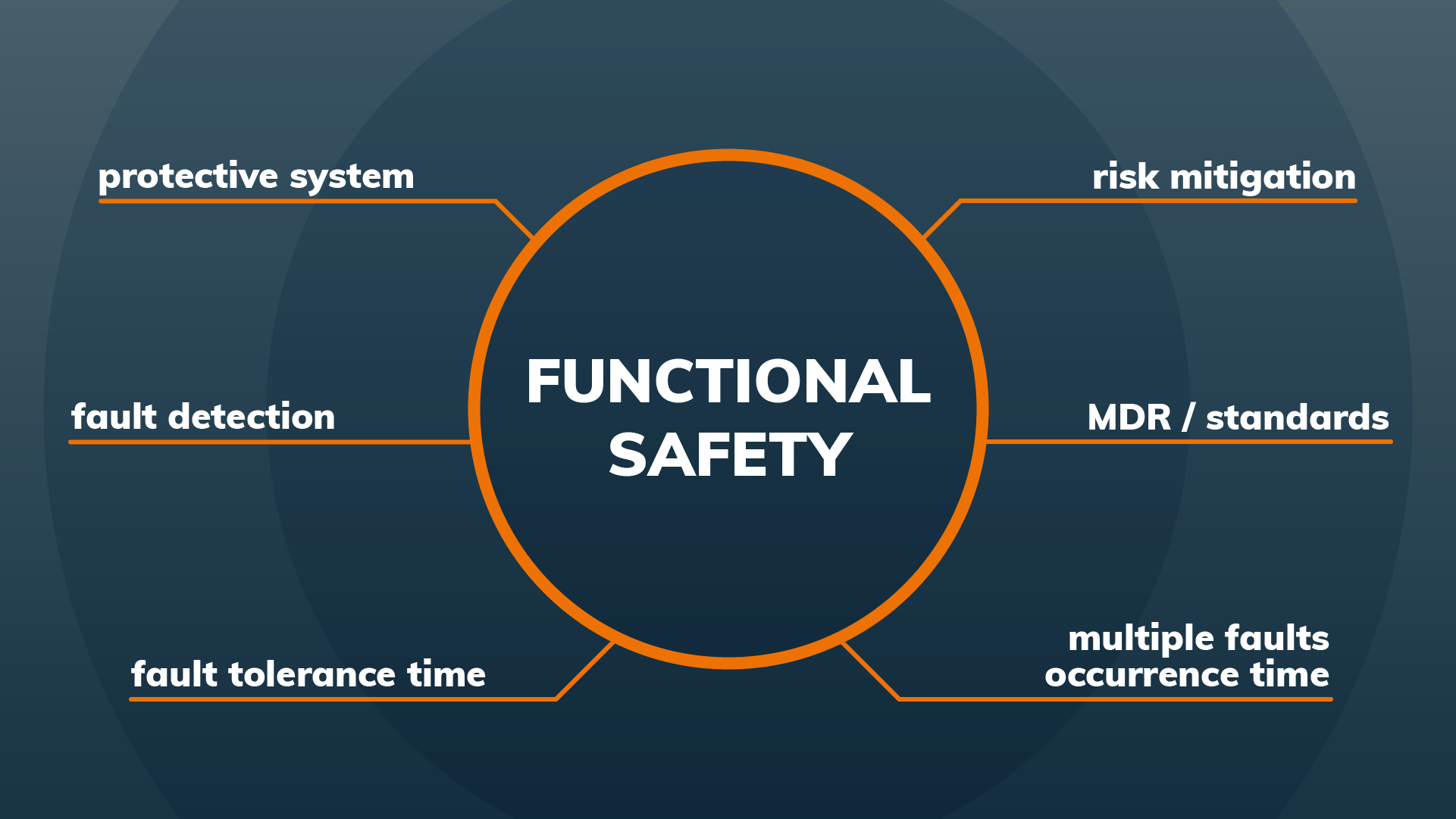
Consulting for medical technology
Quality Management, Regulatory Affairs, Development
In-depth technical know-how, regulatory compliance, tried-and-tested processes: successful MedTech projects have complex requirements. So it is good to have a partner at your side who has been a development service provider and manufacturer of complex, safety-critical medical devices for over 20 years. From conception, project management, and development to transfer to series production – our MedTech experts will be there to advise start-ups and corporations at every stage of the project.
Our MedTech Consulting process in 4 Phases
Research & Development
For the success of your MedTech project
Would you like to have your project checked for feasibility as soon as possible? Are you looking for an experienced partner with whom you can reliably create a Class III medical device? Do you want to implement the phase-gate process and develop more efficiently? Benefit from our many years of experience in development services.
How we support you:
- Requirements engineering (System footprint workshop)
- Proof of concept for hardware and software
- Feasibility studies
- Layout, software and algorithm reviews
- Prefabricated, customizable development solutions
- Insulation diagrams (HV)
- Reviews of complex systems at sub- and system level
- Creating proof of verification
- Cybersecurity concepts
- Test verification and test automation
Quality Management & Regulatory Affairs
With us you can master the MDR approval process
Do you want to transfer a MedTech product from the MDD to the MDR? Do you need help from experts because the regulatory issues seem to be never-ending? As an MDR-certified manufacturer and supplier, we know the processes for approving medical devices inside out and will support you in getting your device approved. Corscience was one of the first medium-sized companies to undergo MDR audit as early as the beginning of 2021. Our experts share their experience and their entire QM/RA knowledge with you.
How we support you:
- Consulting on, set-up or remodeling of a pragmatic
ISO 13485-compliant QM system - Creating and maintaining technical documentation
- MDR gap analyses
- MDR transition
- Approval strategies for active medical devices of all classes
- Approval support for CE, FDA
- Internal mock audits and supplier audits
Start your MedTech project now
As a defibrillation specialist with over 20 years of experience in the development and approval of medical devices, we will find the right solution for you. Make an appointment to clarify first questions.
Our Customers
Success Stories

Download for free
White paper: Label for medical devices
Small surface, big challenge: Labeling medical devices correctly according to current regulations seems like a science in itself at first glance. Our expert Dr. Senem Ntourmas brings clarity to terms like Unique Device Identification (UDI), Labeling and Tracebility. Read her white paper to find out what’s important when it comes to packing all the relevant information about a product into a compact space.